GRAFIX◊ PRIME Cryopreserved Placental Membrane: Significantly more patients with diabetic foot ulcers (DFUs) ≤5 cm2 achieved complete wound closure with reductions in estimated mean costs, compared with Dermagraft™36
Study overview
- Prospective, randomized, single-blind, 9-week, head-to-head study at seven centers in the USA in patients with chronic DFUs (duration: 4-52 weeks; size: 1-15 cm2)
- Patients received either GRAFIX PRIME Membrane (n=38) or Dermagraft (Organogenesis, Canton, MA, USA; n=37) weekly for up to eight applications or until complete wound closure, whichever was first
- Standard of care was wound cleaning and debridement with offloading for plantar DFUs
- More patients in the GRAFIX PRIME Membrane group had plantar wounds and wounds of longer duration, and fewer had lateral wounds, compared with the Dermagraft group (all p<0.05)
- No significant difference in wound size (p=0.732)
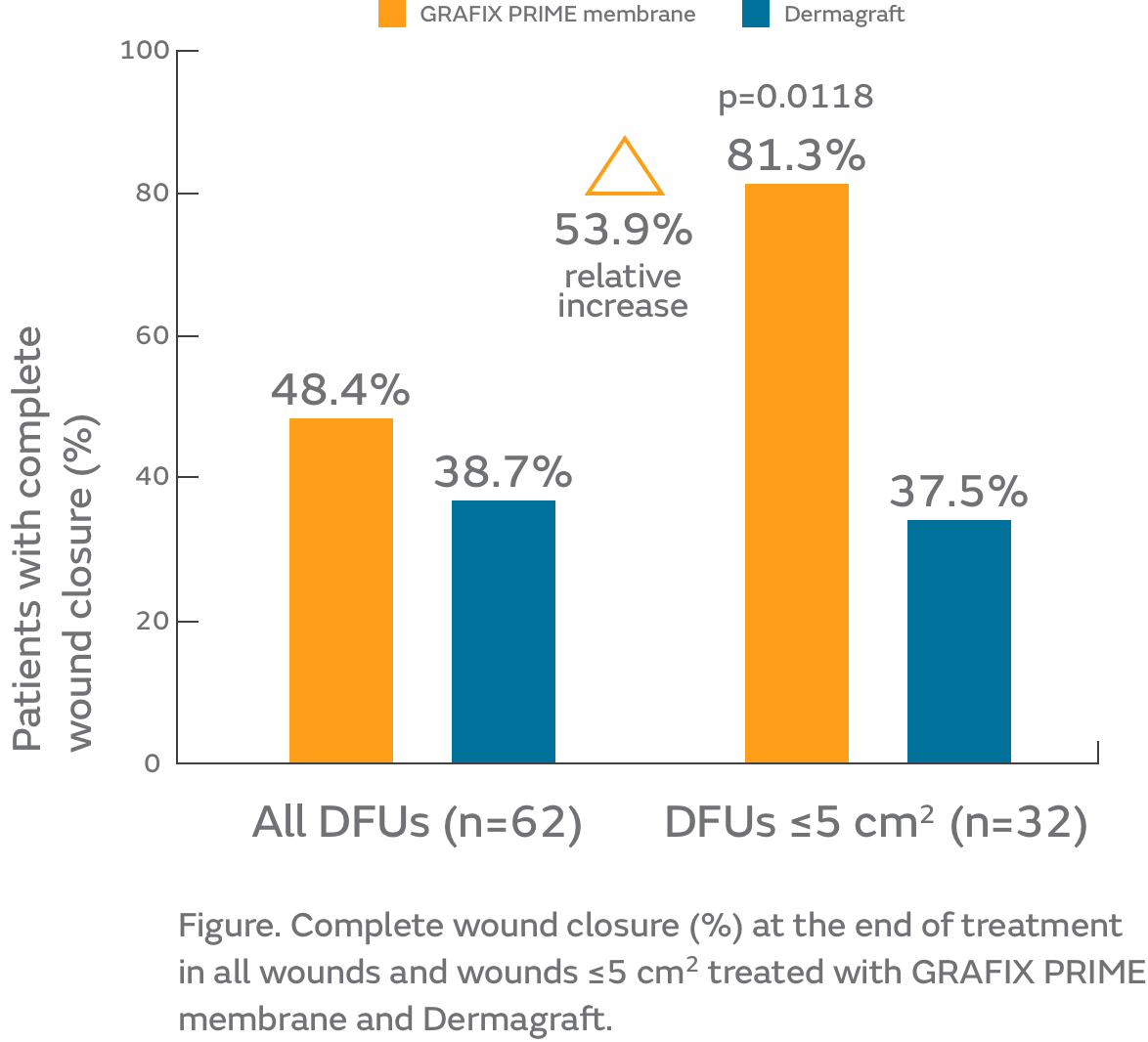
- Of the total population (n=75), 62 patients completed the study (per protocol population) and were included in the non-inferiority, clinical outcomes and cost analyses
Results
- Patients achieving complete wound closure (primary endpoint) was similar (non-inferior) for GRAFIX PRIME Membrane and Dermagraft (Figure)
- For GRAFIX PRIME Membrane compared with Dermagraft there were no significant differences in:
- Wound area reduction ≥ 50%
- Number of product applications
- Mean percentage wound area reduction
- For GRAFIX PRIME Membrane compared with Dermagraft in DFUs ≤ 5cm2:
- Significantly more patients achieved complete wound closure at the end of treatment (p=0.0118; Figure)
- Mean per patient product costs were significantly reduced