CENTRIO Platelet-Rich Plasma System
What is CENTRIO PRP?
A complete point of care system that provides the safe and rapid preparation of a biodynamic hematogel from a small sample of a patient's blood. A single draw provides coverage for wounds up to 2,000 sq cm and the kit includes everything needed – wound dressing, reagents, and phlebotomy tools
Rapid delivery
Minimal amount of blood required
One-minute centrifuge spin
Complete PRP process in under 10 minutes
Keeps pace with your clinic’s schedule
Why CENTRIO PRP?
Some wounds won’t wait. Some clinics can’t slow down. The CENTRIO PRP System gives you what you need to manage both — fast prep, full coverage, and little documentation required for reimbursement, all in four easy steps.

Draw it
Take a small amount of the patient’s blood.
Spin it
Use the CENTRIO System centrifuge to separate the platelets (1 minute).
Mix it
Mix with additives supplied in the CENTRIO Reagent Kit (such as ascorbic acid) to produce a golden biodynamic gel.

Apply it
Apply over the entire wound be and cover with a clear dressing.
A solution that starts and ends with the patient
CENTRIO PRP is ideal for exuding wounds, such as:
- Leg ulcer
- Pressure Ulcer
- Diabetic Ulcer
- For the management of mechanically or surgically debrided wound
The CENTRIO PRP Advantage
Coverage
Covers wounds up to 2,000 cm²
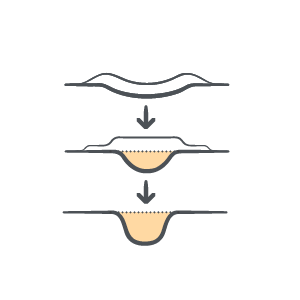
Flexible
Available in flowable gel form to match wound depth and complexity, enabling targeted treatment of sinus tracts, and undermining area/wounds
Versatile
Ideal for diabetic wounds including but not limited to diabetic foot ulcers (DFUs), venous leg ulcers (VLUs), pressure ulcers, and tunneling wounds
Proven
90.5% of wounds treated with an enhanced near-physiological concentration of platelet rich plasma gel showed a 63.6% reduction in wound volume.1
Clinical evidence
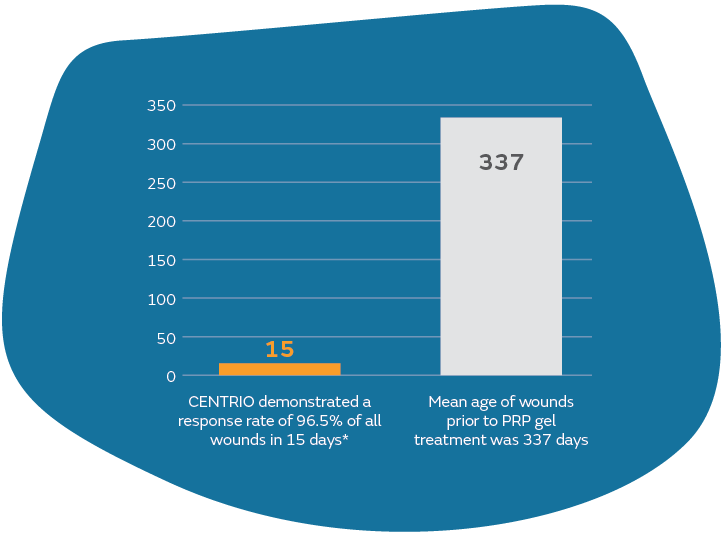
Healing in wounds >337 days old
285 Wounds, 39 Centers, 96.5% response rate in less than 15 days with 2.8 treatments
Prospective, randomized, controlled trial in diabetic foot ulcers (DFU)
81% healed in the most common-sized DFUs

A retrospective, longitudinal study to evaluate healing lower extremity wounds
CENTRIO treatment for three different wounds
Healing in wounds >337 days old
- 85 Wounds, 39 Centers, 96.5% response rate in less than 15 days with 2.8 treatments.
- 90.5% of wounds had a 63.6% volume reduction
- Promotes healing in a wide range of chronic wounds with consistent reduction in area, volume, undermining, sinus tracts and tunneling
*de Leon J, Driver VR, Fylling CP, Carter MJ, Anderson C, Wilson J, et al. (2011) The Clinical Relevance of Treating Chronic Wounds With an Enhanced Near-physiological Concentration of Platelet-Rich Plasma (PRP) Gel. Advances in Skin and Wound Care, 24(8), 357-368
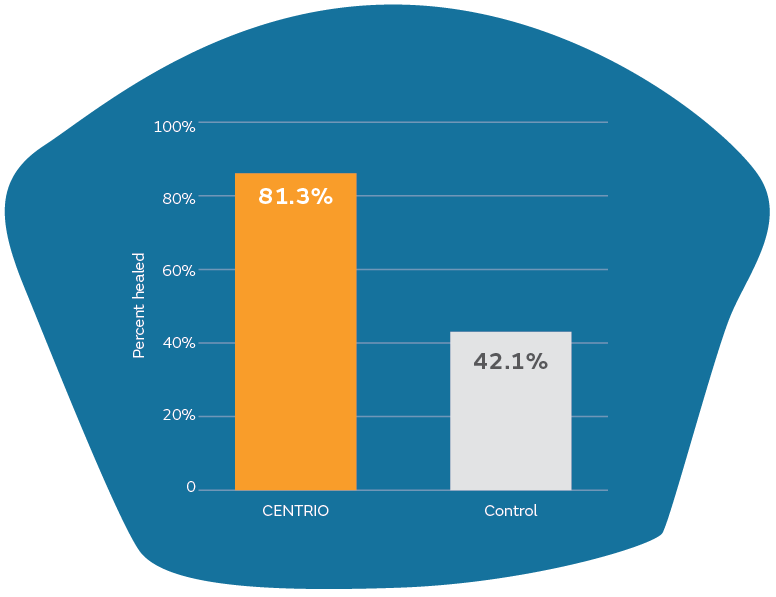
Prospective, randomized, controlled trial in diabetic foot ulcers (DFU)
- 81% healed in the most common sized DFUs
- Significantly more healing (81.3% vs 42.1%) with CENTRIO (p = 0.0364)
- Mean time to healing = 6 weeks
*Driver V, Hanft J, Fylling, C et al. (2006) A Prospective, Randomized, Controlled Trial of Autologous Platelet- Rich Plasma Gel for the Treatment of Diabetic Foot Ulcers. Ostomy Wound Management, 52(6): 68-87.
*Data is for majority wound group with wounds <7cm2 in size
A retrospective, longitudinal study to evaluate healing lower extremity wounds
CENTRIO treatment for three different wounds
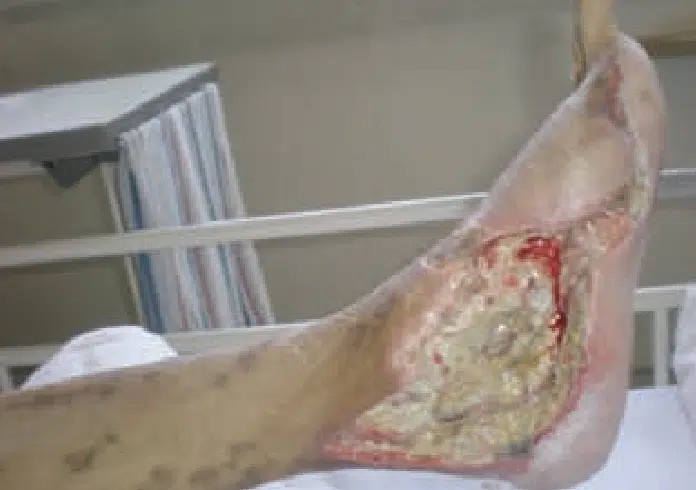
May 4: Prior to CENTRIO treatment. Wound continued to fail despite two revascularizations.
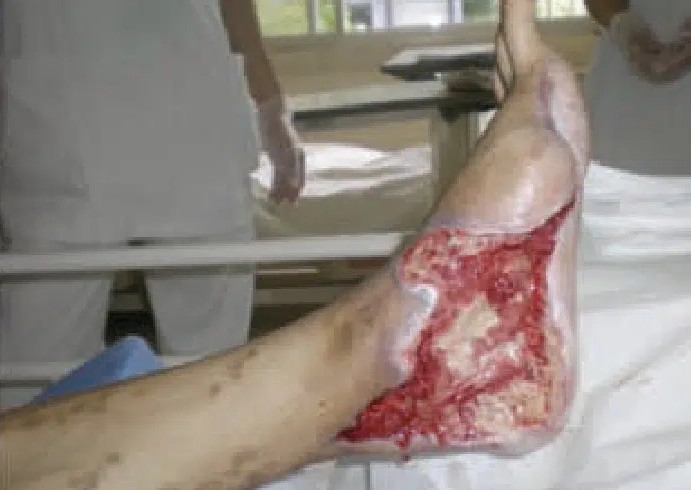
May 26: Wound appearance the day CENTRIO treatment was initiated, post debridement.
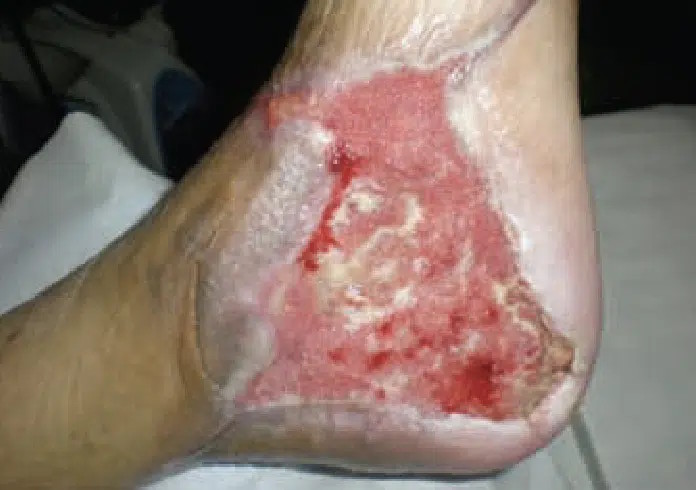
July 7: Wound bed showing granulation tissue with significant reduction in wound area and volume.
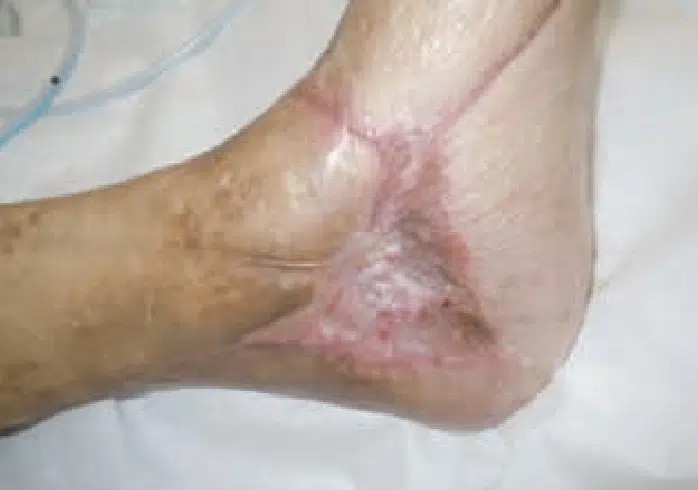
November 4: Full wound closure.
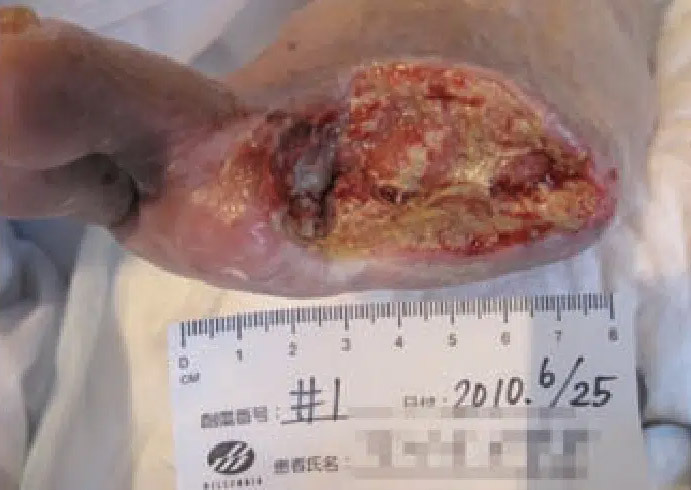
June 25: Diabetic ray amputation site with arteriosclerosis obliterans. Wound not healing despite revascularization.
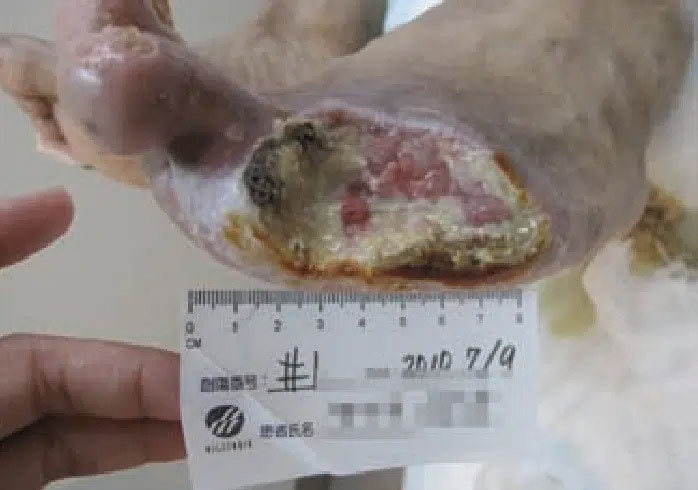
July 9: Wound appearance the day CENTRIO treatment was initiated, pre-debridement.
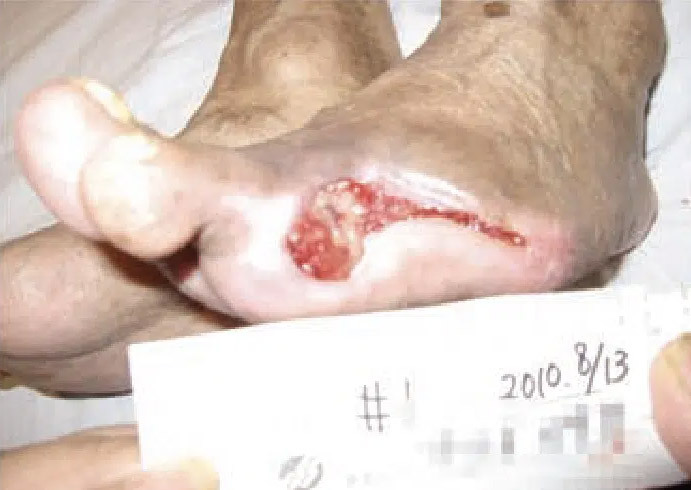
August 13: Amputation site healing well with granulation tissue visible. Significant reduction in wound area and volume.
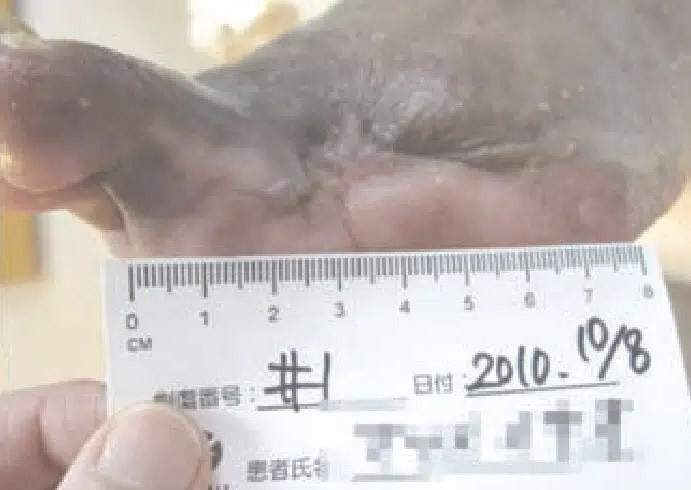
October 8: Wound after 56 days of CENTRIO treatment and one week prior to complete healing.
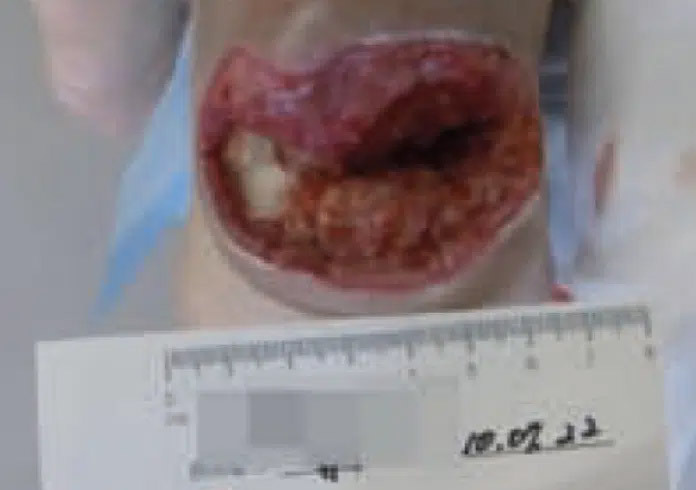
July 22: Prior to CENTRIO treatment. Diabetic heel ulcer with arteriosclerosis obliterans at initial patient visit.
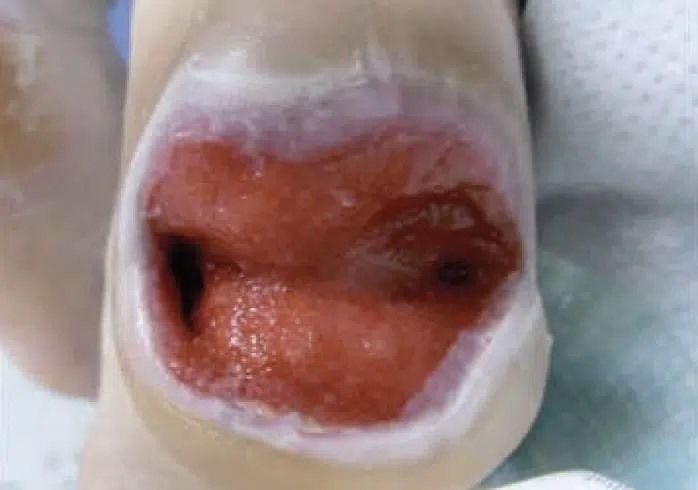
August 12: Granulation tissue visible in wound bed with significant reduction in wound volume.
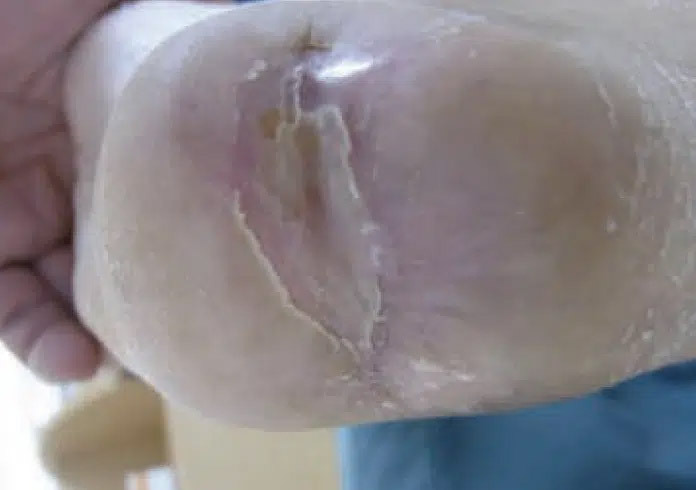
September 30: Wound healed in 49 days of CENTRIO treatments.


