GRAFIX◊ Cryopreserved Placental Membrane: Measurable success in the management of chronic complex diabetic foot ulcers (DFUs)8
Study overview
- Prospective, multicenter, open-label, single-arm trial of GRAFIX Membrane in the management of DFUs with exposed bone and/or tendon
- 31 patients enrolled, 27 patients completed*
Wound characteristics
Wound duration prior to study (mean)
7.5 months
Wound size (mean)
14.6 cm2
Prior advanced wound therapy
67.7%
Results
Wound closure at 16 weeks: 96.3%
- Mean 6.8 graft applications in 6.8 weeks to achieve 100% granulation
Complete epithelialization at 16 weeks: 59.3%
- Mean 9.0 graft applications in 9.1 weeks to achieve complete wound closure
Case example 1†
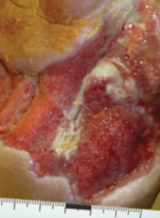
Week 0 wound area: 70 cm2
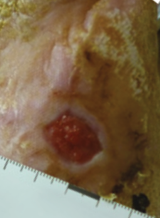
Week 16 wound area: 0.8 cm2
Case example 2†
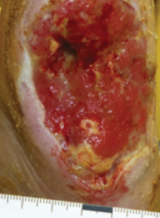
Week 0 wound area: 47.2 cm2
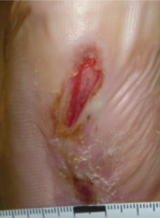
Week 16 wound area: 1.2 cm2
Case example 3†
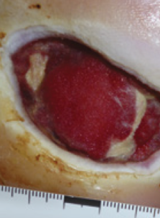
Week 0 wound area: 17.4 cm2
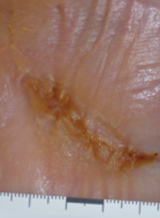
Week 12 wound closure
Case example 4†
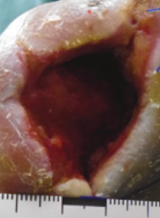
Week 0 wound area: 10.8 cm2
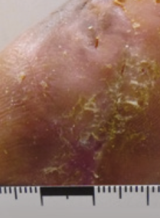
Week 10 wound closure
*Two patients withdrew for non-compliance and two for surgical intervention.
†This series of studies is provided for informational and educational purposes only. These cases may not represent typical outcomes. Every procedure and each patient undergoing wound care treatment represents unique sets of circumstances and, therefore, results may vary. Smith & Nephew does not provide medical advice. The information presented is not, and is not intended to serve as, medical advice. It is the responsibility of the treating physician to determine and utilize the appropriate products and techniques according to their own clinical judgment for each of their patients.

